JPAD:Epidemiology of Hemorrhagic and Gastrointestinal Adverse Effects of Low-dose Aspirin for Primary Prevention of Atherosclerotic Events in Type 2 Diabetic Patients: Insights From JPAD Trial
Takeshi Morimoto1; Hisao Ogawa2; Masafumi Nakayama2; Shiro Uemura3; Masao Kanauchi3; Naofumi Doi3; Hideaki Jinnouchi4; Hirofumi Soejima5; Seigo Sugiyama5; Masako Waki6; Takahiro Kawano7; Yoshihiko Saito8
Takeshi Morimoto1; Hisao Ogawa2; Masafumi Nakayama2; Shiro Uemura3; Masao Kanauchi3; Naofumi Doi3; Hideaki Jinnouchi4; Hirofumi Soejima5; Seigo Sugiyama5; Masako Waki6; Takahiro Kawano7; Yoshihiko Saito8
1 Kyoto Univ Graduate Sch of Medicine, Kyoto, Japan
2 Graduate Sch of Med Sciences, Kumamoto Univ, Kumamoto, Japan
3 Nara Med Univ, Nara, Japan
4 Jinnouchi Clinic Diabetes Care Cntr, Kumamoto, Japan
5 Graduate Sch of Med Sciences, Kumamoto Univ, Kumamoto, Japan
6 Shizuoka City Shizuoka Hosp, Shizuoka, Japan
7 Oyodo Hosp, Yoshino, Japan
8 Nara Med Univ, Nara, Japan; Japanese Primary Prevention of Atherosclerosis with Aspirin for Diabetes (JPAD) Trial Investigators
Objectives: Low-dose aspirin is frequently used for primary prevention of atherosclerotic events in diabetic patients. Because the risk of hemorrhagic and gastrointestinal adverse effects of aspirin was unknown, we analyzed the hemorrhagic and gastrointestinal adverse effects of aspirin in diabetic patients.
Methods: JPAD trial was a randomized, controlled, open-label blinded-endpoint study to examine the efficacy of low-dose aspirin for primary prevention of atherosclerotic events in type 2 diabetic patients. It enrolled 2539 patients with a median follow-up of 4.4 years. Primary end points were all atherosclerotic events and any adverse events were also investigated.
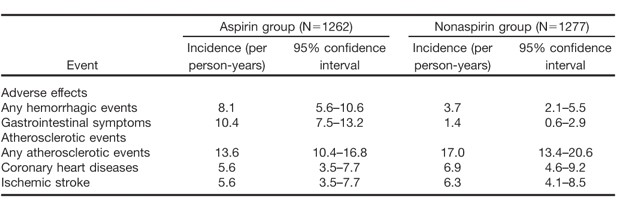
Results: Any hemorrhagic event, including hemorrhagic strokes, consisted of 40 patients in the aspirin group and 19 in the nonaspirin group (p=0.005) and any gastrointestinal symptom in 51 patients in the aspirin group and 7 in the nonaspirin group (p<0.0001). The incidences of hemorrhagic events and gastrointestinal symptoms and atherosclerotic events were shown in Table, and hazard ratio of aspirin for any hemorrhagic event was 2.2 (95%CI, 1.3–3.8) Although there were no fatal cases, 4 patients in the aspirin group had gastrointestinal bleeding which was needed a transfusion. Cerebral hemorrhage consisted of 6 patients in the aspirin group and 7 in the nonaspirin group.
Conclusion: Aspirin was significantly associated with nonfatal hemorrhagic events and gastrointestinal symptoms in Japanese diabetic patients. Although precipitated adverse events were greater than avoided atherosclerotic events, aspirin use for primary prevention should be justified when severities of events were taken into account.
Table. Incidences of adverse effects and atherosclerotic events in JPAD trial



 京公网安备 11010502033353号
京公网安备 11010502033353号